How to share sensitive data: essential tips for researchers
Many publishers encourage authors to share their data as openly as possible to facilitate reuse and support greater transparency of research outcomes. For researchers who have worked with human research participants, however, the request to open their datasets may raise questions. Read on to discover key factors you should consider to share sensitive data legally and ethically.

What makes research data sensitive?
Generally speaking, sensitive research data refers to data that should not be shared in the public domain without additional consideration or permission. This might include human data, data impacting on national security, such as military secrets, or sensitive location data of rare, endangered species or archeological sites, for example.
Types of human data
Depending on the type of research, ‘human data’ can take many forms. In clinical research, sensitive data includes blood samples, tissue samples, genetic sequences, or other health information. In the social sciences, sensitive data can include demographic information, images, videos, audio files, or qualitative data related to attitudes, opinions, or experiences. Plus, many researchers now incorporate datasets originating from social media sites into their research, such as Facebook profiles, tweets, or internet dating profiles, which also constitute human data and sensitive information.
Datasets from human subjects often include personally identifying information, which could allow others to identify research participants if the dataset was released openly. In addition, some research covers particularly sensitive topics, such as alcohol dependency or sexuality, which might lead to increased risk to participants if they were identifiable. Because of these concerns, it’s important that human data is handled appropriately and only shared openly if the ethical and legal implications have been considered.
Understanding legal and ethical obligations
So, what do you need to consider before sharing sensitive human research data?
Essentially there are two key elements you should take into account, the legal and ethical requirements of how the data should be shared and stored. But what does this mean in practice? Let’s take a closer look.
Legal requirements
Firstly, it is important to be aware that legal and ethical frameworks may differ depending on where you are based and where the research is being conducted. You should ensure that you identify and comply with the relevant legislation in your region and discuss any ethical concerns with your Institutional Review Board (IRB) before sharing sensitive data openly. While publishers and funders often have open data sharing policies for researchers, these will take into account sensitive human data and will not require you to share data when it is not appropriate to do so.
Local and regional data protection legislation is usually the basis for most legal aspects of human research data management, data storage, and data sharing. For example, human data is covered by GDPR in the European Union, or HIPAA in the US. You should note that the legislation which applies to your research will usually be determined by where you are based, but if your research participants are in another region then their local legislation may also apply.
Ethical requirements
Ethical frameworks are equally important when it comes to sharing sensitive research data. You should consider the rights and dignity of your participants, ensure that they have given informed consent, and that they have understood how their data will be used and shared. Some research participants may prefer to be identified in a research study, or even find it empowering. You can give participants the option to be identified or have their data anonymized before sharing, as long as they have given their informed consent.
Both legal and ethical requirements are important when it comes to sharing sensitive data and one does not supersede the other. Researchers should take both into account when considering how their research data can be shared.
Approaches for safer sharing
Although researchers should take additional precautions when considering sharing sensitive data openly, there are a number of steps which can be taken to share data safely, ethically, and in compliance with relevant legislation.
#1: Consideration and consent
Before a study begins, even at the point of applying for grant funding, you will need to consider how any sensitive and personal data you collect might be shared. It is important to do this in advance as you should ideally provide an overview of your data sharing plans to your Institutional Review Board as part of the ethical review process. It is also necessary to plan ahead so that when you are recruiting your research participants, you can describe the planned data sharing methodology to them, and obtain their informed consent. To help achieve this, you can consider creating a data management plan (DMP) detailing how you are planning to manage your research data both during and after your research project. In your DMP you can address what types of data you will be collecting and how you will be documenting, storing, sharing, and preserving it.
When approaching potential research participants to join your study, you should provide them with an explanation of what the study will involve, your research purposes, and how their data may be shared. Consent should be written (e.g consent forms) or at least recorded (e.g. an audio file) and it must be given freely – you cannot provide a form with pre-ticked boxes for example. You must also allow participants to opt out of data sharing; or alternatively, they must be able to opt out of the study entirely if they do not agree to their data being shared.
#2: Anonymization
Anonymization removes identifying information from a dataset. It is a method of protecting participant privacy and reducing the likelihood of re-identification. Although anonymization removes the identifiable information from a dataset, you should only apply these processes if you have received informed consent from your participants to do so.
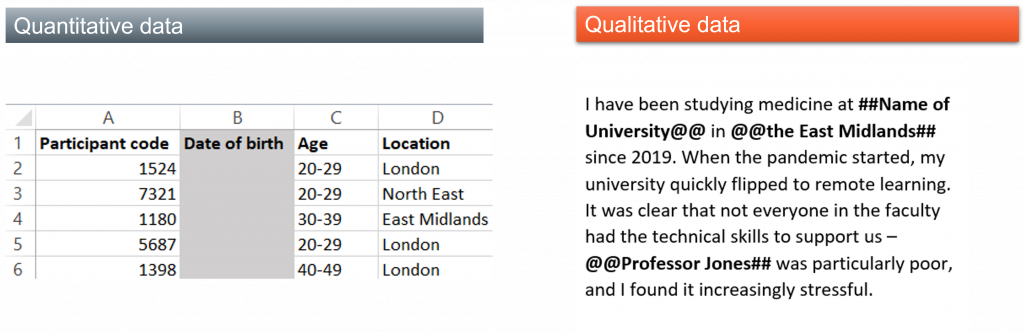
When considering anonymizing a dataset, you’ll likely need to remove both direct and indirect identifiers. A direct identifier such as full name, date of birth, or address uniquely identifies a research participant. Indirect identifiers may uniquely identify a research participant in combination, for example ethnicity, gender, place of birth, and job title. Anonymization is an iterative task which will be specific to the dataset that you generated. You should continually reevaluate the dataset as you conduct the process to ensure that you have removed sufficient information without destroying the value of the data.
Key data anonymization techniques include:
- Removing variables that are not necessary for analysis or relevant to the research, such as IP addresses, latitude and longitude, or date of birth of participants.
- Generalization: making an information point less specific such as swapping an address for a city or a country
- Pseudonymization: referring to a research participant without using their real information by swapping their names for falsified versions
- Creating bands (banding): taking specific information like age or salary and putting it into a range
- Aggregating and reducing variables like age or location to decrease the precision of the variable. Recording birth year rather than the birth date is an example of reduction.
- Logging: keeping a record of each step of anonymization—make sure to keep this file separate from the anonymized data files.
- Reassessing: double checking your dataset following anonymization to remove the risk of disclosure
You may need to use a combination of these when anonymizing a single dataset. Similar techniques can be used for anonymizing qualitative data, and any change to the original dataset can be indicated using diacritics around the text, for example, # or @ symbols.
#3: Controlled access
There may be cases where data cannot be fully anonymized or where the anonymization process would remove so much information that the dataset would no longer be useful. It is still possible to make such research data accessible whilst protecting participant privacy by using a controlled access data repository, as long as you have obtained participant consent to do so.
Controlled access data repositories provide a location, usually on the web, where researchers can store their data, but don’t publish it openly. Examples of such repositories allowing restricted access to protect the anonymity of participants include National Addiction & HIV Data Archive Program, Cancer Imaging Archive, Project Datasphere, or Vivli.
Usually, a metadata record describing the data will be shared openly instead, and the repository will require that users meet certain conditions to ensure that only approved users have data access. If you are publishing a paper based on sensitive data stored in a controlled access repository, you can use your data availability statement to describe where the data is located and the conditions under which it can be accessed.
Openly sharing data may not always be feasible due to ethical considerations or other restrictions. Yet, even sensitive and confidential data can be shared ethically and legally following techniques, such as informed consent, appropriate anonymization, or controlled access. To be successful, the sharing of personal and sensitive data needs to be planned from the outset of a research project.
